Oncology Pipeline
Loxo@Lilly aims to create medicines that make life better for all those affected by cancer around the world. Bringing together the focus and spirit of a biotech with the scale, resources, and heritage of Lilly, our team is focused on rapidly delivering impactful new medicines for people with cancer. Our approach centers on creating oncology medicines that unequivocally show early signs of clinical activity and will matter to patients.
Next-Generation RET Inhibitor
LOXO-260
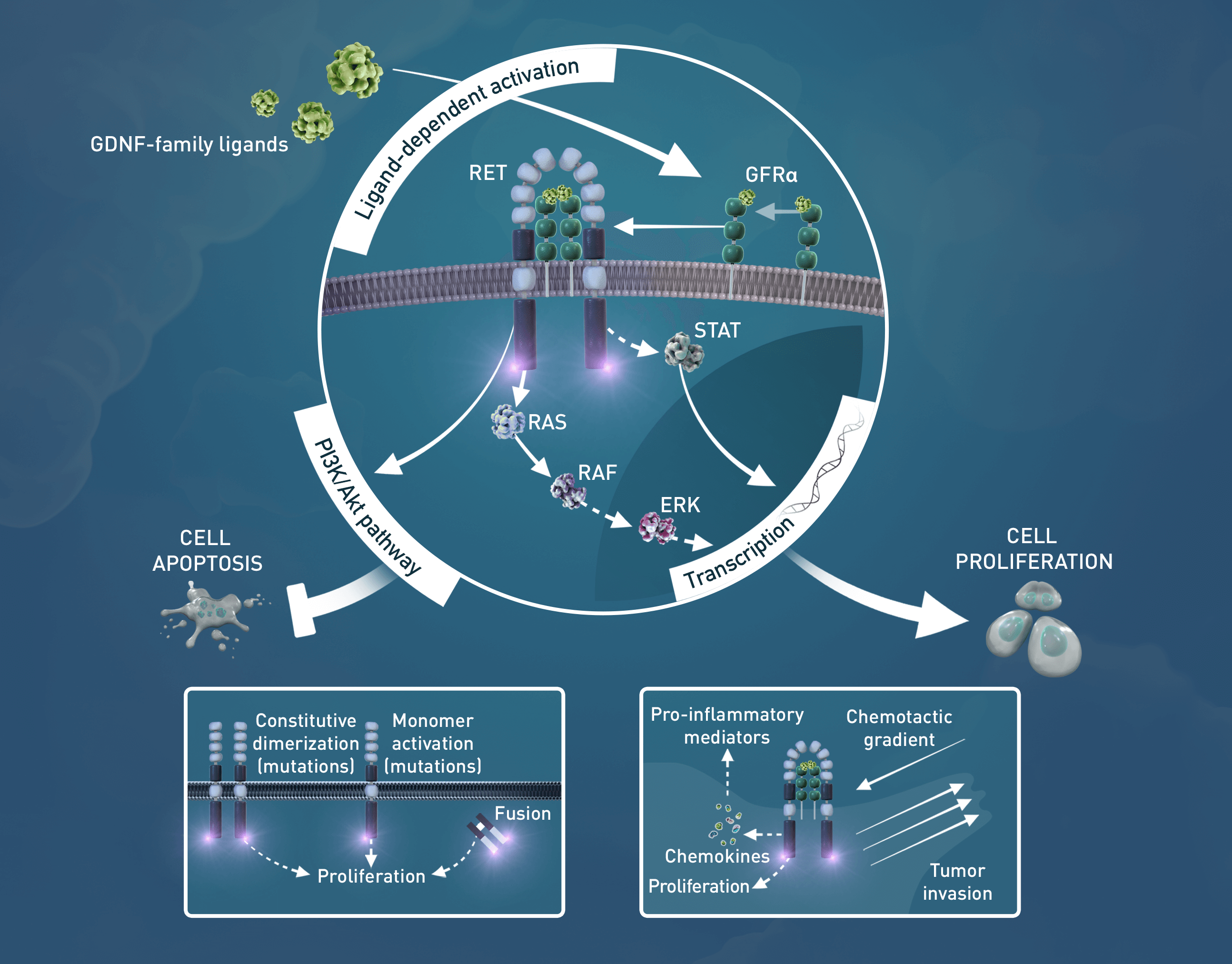
Mulligan LM1
Target
Recently, resistance to targeted RET treatment has been described in the clinic with secondary solvent front mutations or other oncogenic pathway activations emerging.12-14
Molecule
Clinical Development
References
- Mulligan LM. Nat Rev Cancer. 2014;14:173-186.
- Lipson D, et al. Nat Med. 2012;18(3):382-384.
- Takeuchi K, et al. Nat Med. 2012;18(3):378-381.
- Drilon A, et al. Nat Rev Clin Oncol. 2018;15(3):151-167.
- Parimi V, et al. NPJ Precis Oncol. 2023;7(1):10.
- Yang SR, et al. Clin Cancer Res. 2021;27(5):1316-1328.
- Kohno T, et al. Carcinogenesis. 2020;41(2):123-129.
- Li AY, et al. Cancer Treat Rev. 2019;81:101911.
- Hofstra RM, et al. Nature. 1994;367(6461):375-376.
- Agrawal N, et al. J Clin Endocrinol Metab. 2013;98(2):E364-E369.
- Taccaliti A, et al. Curr Genomics. 2011;12(8):618-625.
- Solomon BJ, et al. J Thorac Oncol. 2020;15(4):541-549.
- Subbiah V, et al. Ann Oncol. 2021b;32(2):261-268.
- Rosen EY, et al. Clin Cancer Res. 2021;27(1):34-42.
- AACR disclosure. Kolakowski et al. Pre-clinical characterization of potent and selective next-generation RET inhibitors. Presented at AACR Annual Meeting 2021; April 10, 2021.
Need additional information or have a question:
The safety and efficacy of the agents under investigation have not been established. There is no guarantee that the agents will receive regulatory approval and become commercially available for the uses being investigated.