FGFR3 Inhibitor
LOXO-435
Repetto M, et al1; Chen L, et al2
Target
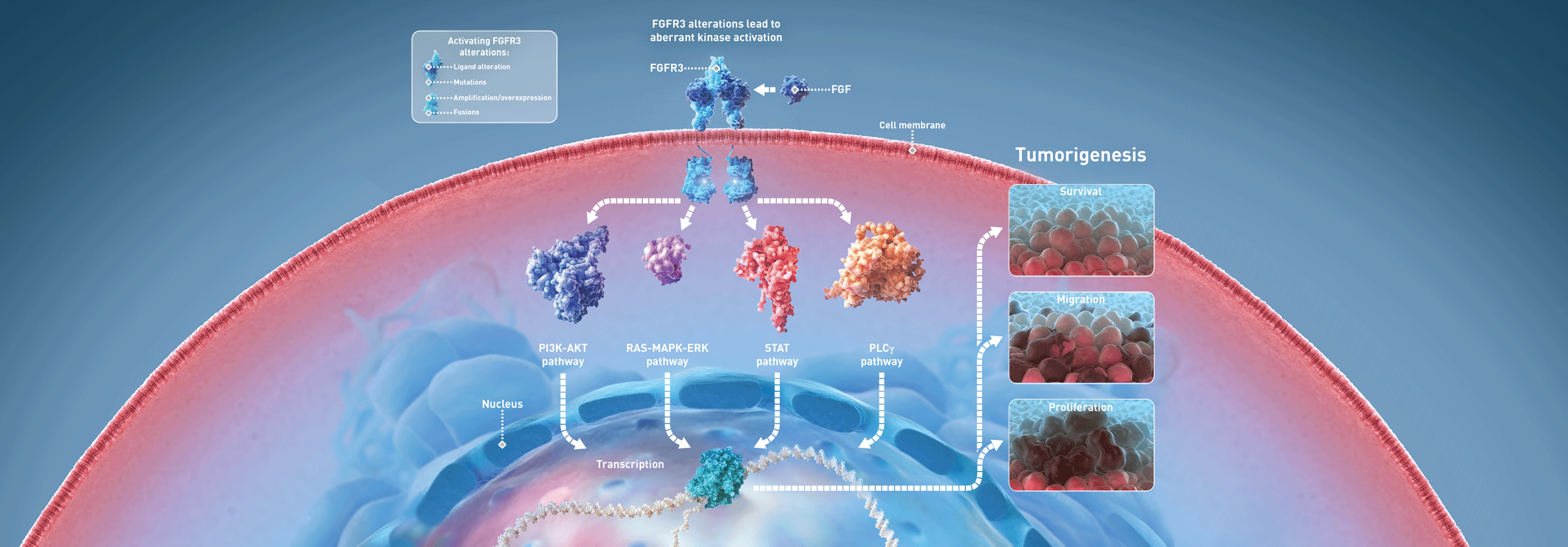
Fibroblast growth factor (FGF) receptor 3 (FGFR3) is a member of the highly conserved FGFR family of transmembrane receptors.2-4 There are four FGF receptors, FGFR1-4, that each consist of an extracellular ligand-binding domain, transmembrane domain, and an intracellular tyrosine kinase domain.3,4 Receptor dimerization induced upon binding of the extracellular domain with a high-affinity member of the FGF family of ligands leads to phosphorylation of the intracellular domain and phospholipase Cγ, PI3K-AKT, RAS-MAPK-ERK, and STAT pathways activation, playing a critical role in several biological and developmental processes.2,4,5 FGFR3 aberrations act as oncogenes across tumor types and have been identified in 15% to 20% of advanced urothelial bladder cancers, ~15% of uterine carcinosarcomas, ~5% of endometrial cancers, and less frequently (<5%) in other solid tumor malignancies.3,4,6,7 Activating FGFR3 alterations are diverse and include point mutations, fusions, amplifications, and overexpression.2-5 Dysregulation of FGFR3 promotes oncogenesis and tumor cell proliferation, migration, and survival.2-5,8
Molecule
LOXO-435 is an isoform-selective FGFR3 inhibitor that has shown antitumor activity across FGFR3-mutant in vivo preclinical models, with preserved potency against FGFR3 gatekeeper resistance mutants.7 LOXO-435 spares FGFR1 and FGFR2 in preclinical in vivo models, with the goal of avoiding dose-limiting hyperphosphatemia and other clinical adverse events that drive chronic intolerance to pan-FGFR inhibitors.7
Clinical Development
LOXO-435 is being investigated in an open-label, multicenter, phase 1a/b study in patients with FGFR3-altered advanced urothelial carcinoma and other solid tumors.
References
- Repetto M, et al. Expert Rev Clin Pharmacol. 2021;14(10):1233-1252.
- Chen L, et al. J Exp Clin Cancer Res. 2021;40(1):345.
- Krook MA, et al. Br J Cancer. 2021;124(5):880-892.
- Katoh M. Nat Rev Clin Oncol. 2019;16(2):105-122.
- Glaser AP, et al. Nat Rev Urol. 2017;14(4):215-229.
- Helsten T, et al. Clin Cancer Res. 2016;22(1):259-267.
- Ballard JA, et al. Mol Cancer Ther. 2021;20(12_Suppl):P141.
- Haugsten EM, et al. Mol Cancer Res. 2010;8(11):1439-1452.
or visit www.clinicaltrials.gov for more information on this trial
